Mental health newsletter, December 2022
By Pernille Bülow, PhD
It's December, which, in my culture, is the month of gift-giving. So the free articles never end!
Here's another one! Be sure to subscribe if you have not already, to learn more about the neuroscience of well, everything cool.
Initially I meant to write about sex and mental health. You know, how sex makes you feel good because your brain has dopamine surges and stuff like that. However, as I was reading articles concerning the neuroscience of romantic relationships, I stumbled upon a remarkable and frankly mind-blowing set of studies that completely detoured my initial intentions. Do not fret! We will address the neuroscience of sex (specifically orgasms), however, in this article we will focus on how semen, yep our old friend semen, might be capable of improving our mental health (without considering all the other benefits of sex).
What do I mean by that? It turns out that semen is more than just little infamous swimmers trying to fertilize eggs. Semen is also made up of various hormones and neurotransmitters that are well-documented to improve mental health. For example, semen includes dopamine and norepinephrine, both of which are important in the treatment of depression. When semen enters the vaginal tract, it may, according to some researchers, affect your brain. For a laugh and some inspiration for funny puns, read this article, which summarizes a set of studies that demonstrate how sexually active females who do not use condoms are generally happier than equally sexual females that do use condoms (Gallup et al., 2002). This difference in wellbeing was not due to relationship-status as both groups were what the (male) authors called “promiscuous”, which, translated into non-oppressive language, means that both groups of females had active, and hopefully fun, sexual lives.
However, a major downfall of these articles is the lack of dissecting the biological plausibility in much, if any, detail. Sure, semen enters the vaginal tract but how does semen influence not just your body but your brain as well? Can it even do that? That’s what we will go into here.
Sadly, few researchers have dedicated their careers to the study of semen and mental health, which means that there are still gaps in our knowledge. I have taken it upon myself to attempt to connect the dots. This means that sometimes I will make educated guesses and come up with hypotheses, i.e. share ideas that have not been scientifically proven yet. But that’s the fun part about science, and I hope you’ll enjoy sharing this journey with me.
For the readers that cannot pass the marshmallow test – aka people that need instant gratification – here is what I learned during my literature search: the goodies in semen are actually biologically capable of activating certain nerve endings in the vaginal tract as well as enter into the blood stream, which, for some of the chemicals, can enter the brain. Thus, semen can, by virtue of its chemical composition, affect the body and brain after entering the vaginal tract. However, it remains unknown whether the goodies in semen have any meaningful effects on your brain activity. Another question is of course the role of sex. Do the goodies in semen only really have an effect if they are delivered via sex or could we just “dump” some semen into our vaginal tracts to make us feel better? Theoretically the latter is possible, but this has gone untested. Therefore, in the context of this article, when I talk about the positive effects of semen, I talk about it as a part of sexual intercourse. And just one more reminder: what we discuss here is the possibility of semen improving your mental health. And hopefully a scientist who reads this will start conducting actual experiments to address these dire questions.
With that, let’s get to it.
Definitions
In this article, I will be referring to vaginal tracts a lot. I link the vaginal tract to the female body, but this does not mean that I assume a person with a female body also holds a female identity Anyone with a vaginal tract will benefit from the semen, and if you are not lucky enough to have a vaginal tract then there are other effective routes of entry for our new best friends. You can probably guess those already but rest assured that we will take a holistic perspective on how semen can enter and impact your body and mental wellbeing, regardless of which sex you were born with and what gender identity you hold.
What is the chemical content of semen?
Let’s first take a moment to talk about the chemical composition of semen.
You may already know this, but only a small fraction, around 2-5%, of semen is made up of the eager little swimmers known as sperm cells, or in fancier terms: spermatozoa (Vitku et al., 2017). The rest is the ‘non-impregnating’ seminal fluid, which could be misunderstood as being non-important. However, quick side-track: sperm cells cannot immediately fertilize an egg (Ramirez-Reveco et al., 2017). The sperm cell needs to go through several stages of biochemical processes, referred to as ‘capacitation’, after it has entered the vaginal tract. This process is dependent on the chemicals found in seminal fluid. We will come back to this momentarily.
Researchers have analyzed the chemical content of both spermatozoa as well as seminal fluid, and they have, needless to say, found some interesting “stuff”. While sperm cells are interesting, I will focus on seminal fluid here, mainly because seminal fluid makes up most of semen. Seminal fluid contains so many goodies (summarized in Figure 1) (Vitku et al., 2017). Of these, dopamine, norepinephrine, prostaglandins, oxytocin, progesterone, estradiol, and estrone are previously associated with mood and mental wellbeing. Something that is really cool is that the Nerve Growth Factor (NGF) in seminal fluid can trigger ovulation in female mammals…. In other words, the act of receiving semen into the vaginal tract can initiate ovulation, or what a friend of mine calls “egg-dropping”, which, presumably, enhances your chance of becoming pregnant at the “next visit”.
Now I need you to recall that sperms cells need to be ‘capacitated’ before they can fertilize and egg. What did I mean by that? During capacitation, the sperm cells undergo several changes that enable it to enter an egg. This process is dependent on most, if not all, of the neurotransmitters and hormones found in seminal fluid. Indeed, sperm cells express receptors that can bind for example dopamine which triggers a transformational cascade inside of the sperm cell. In fact, if the dopamine content in seminal fluid is low it reduces sperm mobility and lower fertility (Kanno et al., 2022; Angelis et al., 2016; Ramirez et al., 2009). Likewise, if oxytocin levels are off, it impairs the mobility and number of our enthusiastic swimmers (Vitku et al., 2017; Fuchs et al., 1989). Thus, the contents of seminal fluids are extremely important for determining how effectively sperm can fertilize an egg.

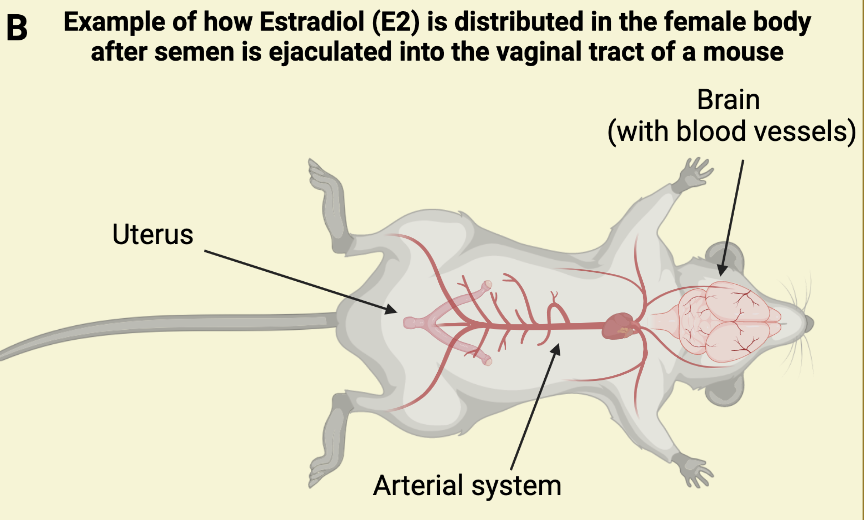
Figure 1:
A: A list of some of the hormones and the two major neurotransmitters found in seminal fluid (NGF = Neural Growth Factor; FSH = Follicular Stimulating Hormone). Note that this list is not exhaustive, by excluding many nutrients, e.g. glucose and zinc, as well as some steroid hormones. You can read more about the contents of seminal fluid in this paper (Vitku et al., 2017). Chemicals written in yellow cross the blood-brain barrier, while the neurotransmitters (written in white) do not. We talk about the blood-brain barrier in the paragraphs below.
B: In the yellow(ish) quadrant you see an illustration of the organs where radiolabeled estradiol was identified after sexual intercourse between two mice ending in ejaculation (deCatanzaro & Pollock, 2016). Radiolabeled estradiol was identified in the brain, blood, and uterus 20 minutes, 3 hours, and 18 hours after sexual intercourse. This research implies that the goodies in semen can have systemic effects on the body, that are not only localized to the vaginal tract and uterus.
Do the semen-goodies really pass into the rest of the body after reaching the vaginal tract?
The short answer is yes.
One research group used a technique to tag all the estradiol hormones in semen of male mice with a radioactive label which they could visualize under a microscope (deCatanzaro & Pollock, 2016). This labeling technique allowed the researchers to see where the estradiol hormones in semen travelled after intercourse with a female mouse. And lo and behold, the scientists did find radioactive labeling (meaning that they found estradiol) in not just the uterus of the female mouse, but also in the blood and brain both shortly after mating had completed, as well 3- and 18-hours post-intercourse. Somehow, the radioactively labeled estradiol in semen spread not just into the uterus but also into blood circulation and through the blood-brain barrier to the brain (we will talk more about the blood-brain barrier in the next sections). Estradiol is important for triggering a surge of luteinizing hormone (LH) via the hypothalamic brain region, and ultimately the process of ovulation (Schjenken and Robertson, 2020). More studies are needed to assess if the amount of estradiol that passes the blood-barrier after semen enters the vaginal tract is large enough to realistically trigger the LH surge and ovulation, but it is interesting to speculate if this is how semen can affect ovulation patterns in females (Schjenken and Robertson, 2020). Interestingly, estradiol is also associated with mental wellbeing, which is most clearly demonstrated in post-partum depression and post-menopause which are both associated with large reductions in estradiol (Watson et al., 2010). Interestingly, estradiol can increase release of dopamine neurotransmission (Barth et al., 2015), which may be one of the major mechanisms through which estradiol improves mood. It is important to note that the positive effects of estradiol are often only associated with higher than physiologically normal levels circulating in the blood – but could those higher levels be provided through semen? Maybe, maybe not. Future research will have to address that question.
The fact that estradiol enters the blood stream is in itself of great interest. Via the blood, estradiol can access the entire body and modulate different organs. If this is true for other hormones and compounds found in semen, it implies that ejaculated semen can have systemic functions on our physiological state, for example by modulating the activity of our sympathetic and/or parasympathetic nervous systems, both of which contribute to our physical and mental wellbeing.
So it seems pretty clear that hormones and neurotransmitters in semen can travel into and throughout the female body, potentially with consequences for not just fertility but also immediate sensations and long-term wellbeing.
But how does this magical goo enter the body and brain via the vaginal tract?
How do the semen-goodies reach your brain – and what might they do?
The vaginal tract and uterus are lined by a mucous membrane that, as most people with female-bodies are intensely familiar with, cycle through different stages every month. When the uterine mucosa sheds, we menstruate. I will explain two distinct routes of semen entry via the vaginal tract, and I will start with the one that relies on our mucosa.
Route 1: semen-goodies diffuse into the blood stream
One of the ways the compounds in semen can pass into the body is through the wonderful mucosa via simple passive diffusion (meaning it does not require any energy or additional molecules for the compounds in semen to pass through the mucosa). The mucosa is porous which allows easy absorption, and importantly, because the compounds bypass the liver, they are not metabolized and thus not degraded in any way (Nicole, 2014). This is the reason that delivering drugs through the vaginal (as well as anal) tract often act more effectively and rapidly, compared to delivering through the mouth.
Now, what happens after they have entered the blood stream? A chemical can of course affect organs and other peripheral tissues directly, which can have great implications for how you feel. But to get into the brain, the compounds would have to pass the blood-brain barrier. The blood-brain barrier, also referred to as BBB, is a mesh of tissue and blood vessels that separates the brain from compounds circulating in the rest of the body, to ensure that pathogens and other “bad stuff” does not enter our precious brain (Sweeney et al., 2018). Only certain types of chemicals can pass through the blood-brain barrier, and these are usually small with a specific chemical composition that I won’t go into here. Certain genetic mutations, like mutations found in people with Alzheimer’s Disease, can cause the blood-brain barrier to break down leading to pathological molecules passing from the body’s blood circulation into the brain, which may contribute to disease progression (Sweeney et al., 2018). However, let’s turn our focus back to the more light-hearted topic of semen and mental health.
As I mentioned, estradiol can pass through the blood-brain barrier. Tyrosine hydroxylase (TH) – which is an important building block for dopamine and is also found in seminal fluid – can also pass the blood-brain barrier. Now, do recall what I mentioned about capacitation of sperm cells. TH is an important participant in this process as well as for ensuring that the sperm cells are speedy, but it is possible that the ‘leftover’ TH could diffuse through the vaginal mucosa into the bloodstream, pass the blood-brain barrier, and ultimately lead to increased dopamine production in neurons. Together with estradiol, this may lead to a longer-term increase in dopamine release after sexual intercourse (and not just an acute sensation of feeling good).

Figure 2: The goodies in semen can, hypothetically, reach the brain via two different routes. Either via binding to neurotransmitter receptors in the vaginal tract or endometrium (which lines the uterus), resulting in activation of a local circuit in the spinal cord and brain. Alternatively, the goodies are absorbed through the mucosa of the vaginal tract, which leads into the bloodstream from where the goodies can affect the entire body. If the goodies make it up to the head region, they will have to pass through the blood-brain barrier before entering the brain. Almost all steroid hormones, like estradiol, can pass the blood-brain barrier, but larger compounds, such as the neurotransmitters dopamine and norepinephrine, cannot.
Route 2: semen-goodies activate receptors
But there is another way that the goodies in semen can affect the female body: receptors (read about neurotransmitter receptors in the Intro to the Brain document). Several types of nerve-endings innervate the vaginal tract and the endometrium. For example, nerve endings expressing dopamine receptors are present in the endometrium (Novella-Maestra et al., 2010) and estrogen receptors are present in the vaginal tract and, to a lesser extent, in the endometrium (Wang et al., 2010; Press et al., 1986). This means that estradiol can affect the central nervous system (which includes the brain and the spinal cord) via two routes: both by passing the blood-brain barrier as well as by binding to receptors present in the vaginal tract. In contrast, dopamine which is too large to pass the blood-brain barrier, can only affect the brain by binding to the receptors in the vaginal tract. While it is unknown whether semen actually activates these receptor pathways, we do know that the biological potential is there.
One important biological note is how far semen actually goes into the vagina. According to studies, most of the semen hangs out in the vaginal tract and accumulates in the cervix. A much smaller percentage makes it all the way into the uterus, which is why it is statistically quite difficult to get pregnant. This also means that it is less likely for the dopamine receptors to be activated compared to the nerve endings binding estradiol and norepinephrine.
The route of entry matters
The speed and location(s) of brain activity will depend entirely on the route of entry.
When estradiol enters the brain via the blood-brain barrier it is systemic and therefore able to have effects anywhere in the brain. In contrast, when estradiol binds to receptors in the vaginal tract it leads to localized activation of specific brain regions. In estradiol’s case, these nerve endings may only reach the hypothalamus which will mainly affect the female’s reproductive cycle. I could not find any research addressing exactly where the nerve endings with dopamine receptors relay their information, but one solid guess is that it reaches the motor cortex or basal ganglia which are important for muscle movements. Remember that your vagina muscles are contracting during sex! On the other hand, when TH, the building block for dopamine, enters the brain through the blood-brain barrier it could have effects in “reward” areas that are known to make you feel good. Now, my favorite example is how the neurotransmitter norepinephrine can affect the brain.
Just like dopamine, norepinephrine (NE) is too large to pass the blood-brain barrier (and thereby enter the brain) but it is the only neurotransmitter which can activate a particular subset of receptors on sensory neurons that upon activation create a sensation of “gentle touch” (Hoffman et al., 2018). Curiously, these nerve-endings with “gentle touch” receptors innervate the vaginal tract (Polakovičová et al., 2018), meaning that they may be directly activated by NE in seminal fluid which could activate the areas of the brain that perceive touch, the somatosensory cortex – a brain region that is connected to many others including your reward aka feel-good regions. This may be one of the biological mechanisms for why some people with vaginal tracts really enjoy the feeling of condom-less ejaculation.
In summary: there are two routes through which semen could positively affect a person’s mental health, either through the blood-brain barrier or through receptors populating the vaginal tract. Whether the semen-goodies have meaningful effects on our mental health is yet to be uncovered.
But the biological potential appears to exist.
For how long could these semen-induced changes last?
You might be wondering how much condom-less sex you need to maintain for these (possible) positive effects to emerge. Obviously, we cannot address this question yet since we do not know whether the goodies have an effect in the first place. However, if they do have a positive effect on your mental wellbeing, then I would be very curious to know whether semen changes phosphorylation status of distinct proteins in the brain. Phosphorylation is a process where a phosphate (just think of it as a compound of molecules) attaches to a protein, for example a receptor. De-phosphorylation is the inverse, which means that a phosphate was removed from a protein. Why am I suddenly giving you a biology lecture? Because (de-)phosphorylation is a common and effective way to create long-term changes in how active, sensitive, and persistent a certain protein is. For example, phosphorylation is necessary for the sustained anti-depressant effects of ketamine (Kim et al., 2021; a drug we will talk much more about in future newsletters!). Unfortunately, this possibility remains completely unexplored.
Of course, we know from one of the first studies I mentioned to you (deCatanzaro & Pollock, 2016), that the radiolabeled estradiol found in semen persisted in the body and brain of the female for up to 18 hours after intercourse (with ejaculation). In human samples, spermatozoa can persist for up to several days as they hang out waiting for an egg to drop (Suarez and Pacey, 2006). But it’s really not that important how long the goodies in semen stay in the woman’s body, the critical aspect is what they do while they are there. In fact, it’s well-established that unused semen will start the process of degradation within the woman’s vaginal tract after a few days (and much of it of course leaves her vagina automatically due to gravitation after sex). Thus, I am not too concerned about how long the goodies in semen hang out, but rather if they can induce long-lasting changes such as phosphorylation.
But to be safe, maybe aim for sex once a day? You never know. The stuff in semen could theoretically be the gamechanger for how you perceive your day. Is it wet and gray or is it an opportune moment to dance in the waterfall from the sky?
What about other ways of having semen delivered?
When I first read about how semen has the potential to positively impact our mental health, I thought about whether this would also be true if we received semen through other routes, say orally or anally.
The oral route
If you receive semen orally, popularly known as “swallowing”, then the semen will enter your mouth travel through your esophagus and then enter your stomach, where the nutrients in semen are absorbed into the bloodstream to get metabolized by the liver. Here semen’s excellent nutrients, like zinc and magnesium, might get distributed to the rest of the body. Like a vitamin pill would.
Have you heard of the gut-brain axis? It is a reference to how the central nervous system (i.e. your brain and spinal cord) communicate directly with your enteric nervous system (which includes your gut and colon) ultimately linking your brain activity, affected by emotions and thoughts, to your intestinal functions (Carabotti et al., 2015). The way this ‘linking’ occurs is through nerve endings innervating the gut and colon. These nerve endings express various receptors, including receptors that bind to dopamine and norepinephrine (Mittal et al., 2017). Thus, when you consume semen orally, dopamine and norepinephrine from semen could have direct effects via your gut on your brain activity that may improve your mental well-being.
It is not just the gut-brain axis that makes the gut cool. The gut also has a ‘microbiome’ which refers to all the microorganisms that live in your gut. The gut microbiome has received a lot of research interest lately, especially from neuroscientists, because we are learning that the composition of the gut microbiome can have consequences for your brain and behavior. For example, abnormalities in the gut microbiome have been linked to depression and autism spectrum disorders (Taniya et al., 2022; Pierce and Alviña, 2019).Though completely unknown, it is interesting to wonder whether oral consumption of semen can alter out gut microbiome in a way that benefits our physical or mental wellbeing. Indeed, the gut microbiome of a male, can influence their own sperm production and thus fertility (Wang and Xie, 2021).
In other words, semen could theoretically function as a vitamin pill and an antidepressant all at the same time. That’s a pretty powerful mouthful!

Figure 3: the gut-brain axis represents a bidirectional communication system between the gut/intestines and the brain. The gut, which we focus on here, is innervated by nerve endings that express receptors that bind dopamine. When dopamine enters the gut, for example via swallowing semen, this could lead to fast activation of the nerve endings, and ultimately specific regions of the brain. The gut-brain axis is still a newer phenomenon in science, so there are many questions to explore in this field. However, we do know that ingesting drugs such as antidepressants activate nerve endings innervating the gut which also explains why antidepressants can make you feel nauseous and/or change your appetite. Researchers are currently performing experiments to test whether antidepressants may also alter one’s mood by acting on receptors in the gut.
The anal route
Another way to receive a dose of this organic goo is via anal sex. The rectum, which is there the penis “hangs out” during anal sex is lined with mucosa and has lots of blood vessels surrounding it (DeBoer et al. 1982). It is actually very common for medical doctors to deliver drugs via the rectum simply because the absorption is better and faster than through oral delivery. The rectum is also densely innervated by sensory neurons, and rectal stimulation (for example by distension or pain) can activate specific brain regions, like the insula and anterior cingulate, in humans (Brierley et al., 2018). This is a part of the gut-brain axis (Figure 3) and implies that dopamine and/or norepinephrine could activate nerve endings in the rectum causing brain activity. In fact, studies have found that when norepinephrine binds to receptors in the rectum it leads to a local release of serotonin which causes hypersensitivity in the rectum (Brierley et al., 2018). It is untested if compounds in semen could activate any nerve endings in the rectum, but there does seem to be a biological potential.
Hypothetically, semen could also influence our bodies through the rectum by passively diffusing through the mucosa into the blood stream. Just like with the vagina, this route can quickly spread through the entire body and potentially have global effects of the brain if the compounds pass the blood-brain barrier.
In conclusion: Whether your preferred delivery method is through the vagina, rectum, or mouth, you are certain to get the good benefits semen has to offer.
Semen – the next frontier
Imagine a world where you are prescribed a regular dose of semen to boost your mental wellbeing. Maybe semen provides the secret recipe for a future antidepressant that organically boosts not just your brain’s but also your body’s health. Do keep in mind that many antidepressants are likely already acting through the gut-brain axis, simply because there are so many serotonin and dopamine receptors in the gut and that’s where the little pill first lands after ingestion. But maybe absorption through the vaginal tract is the next step in optimizing antidepressant delivery? Perhaps the semen could be delivered via a tampon-looking device that would slowly release the goodies of semen to make you feel better not just today, but also in a month.
You can tell that I hold high hopes for semen.
I also hold high hopes for orgasms. A topic we will address next month in the Neuroscience Newsletter.
Conclusion & Disclaimers
Successful sperm cells fertilize a woman’s ovulated eggs, and seminal fluid contains critical nutrients, hormones, and neurotransmitters to enable sperm’s motility and ability to fertilize. And that’s great. But that left over sperm and seminal fluid may be more than a reason to take a sprint to the bathroom. In fact, you might want to practice hand stands after sex to help that magical goo along its fancy way.
While much more research is needed, it is intriguing how the female body appears to have the biological potential to take advantage of the goodies in semen. This may happen through activation of receptors in the vaginal tract and uterus, or by entering the blood stream. Research is needed to clarify whether either of these routes are actually activated by semen.
If semen can affect the female body, then researchers need to test if the concentration of goodies in semen are high enough to have a measurable impact on our wellbeing, whether that be specifically in the brain or in the rest of the body. Moreover, research would need to test how long these effects last. According to early studies (Gallup et al., 2002), females that practiced unprotected sex were happier than their equally sexually active female friends that used condoms. This research is incredibly interesting but calls for many more follow-up studies to figure out if semen could be the new antidepressant.
On that note, practicing safe sex is paramount and I encourage you all to do so regardless of how curious you may be regarding the happiness-inducing potential of semen. Perhaps focus on testing the hypothetical benefits of semen through the oral or rectal routes that do not warrant possible pregnancies.
Lastly, an interesting omission in the original study uncovering the role of semen in mental wellbeing (Gallup et al., 2002) is whether birth control (e.g. the pill or IUD) will experience similar semen-inducing benefits as women that are ‘clean’. Most birth controls increase the cervical mucus which could potentially hamper the goodies’ ability to bind to receptors or enter the blood stream. Again, more research is needed to address these questions.
Cool stuff you don’t need to know, but probably want to know
Did you know that…
- …Your vagina has an immune response to new semen (i.e. semen from a new penis-owner), and over time develops immunity, which makes your uterus tolerate the semen better (Schjenken and Robertson, 2020; Robertson and Sharkey, 2016). Not only does this mean that your vagina can differentiate semen from different men (how cool is that?), but it is also an important step for impregnation. Exposure to seminal fluid is important for a healthy pregnancy because it reduces the female’s immune response to the fertilized egg. In fact, it is more typical for female bodies to experience problems during pregnancy if they have conceived after limited exposure to the seminal fluid of the penis-owner, and in animal experiments, lack of seminal fluid exposure during impregnation results in a higher rate of altered growth, metabolic disease, and even anxiety in the offspring (Robertson and Sharkey, 2016). I think this immune tolerance is really cute, because it basically suggests that your vagina has to get used to semen over time. Almost as if they are developing a romantic relationship of their own.
And that…
- …Researchers developed a theory called the ‘sperm competition’ or ‘semen displacement’ hypothesis which posits that one man’s semen can displace another man’s semen within a vagina (Gallup and Burch, 2004; Parker, 1970). In other words, if a female has sexual intercourse with two different males within a short time-period (a few days), then the second male’s penis has the capability of dislodging and ‘removing’ semen left behind from the first man. How the hell would that be possible? The human penis apparently has distinct anatomical qualities that enables it to ‘dig out’ previous sperm. However, the scientific evidence for this theory is lacking, and it also poses the issue of whether they may potentially dig out their own sperm from a previous intercourse? That seems evolutionarily stupid. Some researchers have countered that problem by posing the idea of ‘killer-sperm’ in the ‘inter-ejaculate sperm competition’ hypothesis (Baker and Bellis, 1988). Yep, you got it right: killer-sperm. These killer machines are supposedly designed only to kill foreign spermatozoa, which obviously would align well with the ‘sperm competition’ hypothesis’ overarching idea of sperm displacement from another male. Sadly, despite the killer-sperm idea sounding killer-cool, this theory has been disproven in subsequent research studies (Moore et al., 1999). What we do know is that the more aroused a penis-owner is, the greater volume of semen is ejaculated (Pham et al., 2016). Yeah, a pretty boring and ‘whatever’ fact, except if you are trying to get pregnant then it’s actually a really important point.
I hope the contents of this newsletter has inspired you!
Happy holidays!
References:
De Angelis, C., Cariati, F., Galdiero, G., Coppola, G., Galdiero, M., Salzano, C., Pivonello, C., Patalano, R., Alviggi, C., De Placido, G., Colao, A., Pivonello, R. (2016). The role of dopamine pathway on human sperm: in vitro effect of dopamine receptor agonists and antagonists on sperm motility, kinetics and viability. Presented at Society for Endocrinology ECE 2016
Baker, R.R., Bellis, M.A. (1988) ‘Kamikaze’ sperm in mammals?, Animal Behaviour, Volume 36, Issue 3, Pages 936-939, ISSN 0003-3472, https://doi.org/10.1016/S0003-3472(88)80178-7.
Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015 Feb 20;9:37. doi: 10.3389/fnins.2015.00037. PMID: 25750611; PMCID: PMC4335177.
Brierley SM, Hibberd TJ, Spencer NJ. Spinal Afferent Innervation of the Colon and Rectum. Front Cell Neurosci. 2018 Dec 4;12:467. doi: 10.3389/fncel.2018.00467. PMID: 30564102; PMCID: PMC6288476.
Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015 Apr-Jun;28(2):203-209. PMID: 25830558; PMCID: PMC4367209.
de Boer AG, Moolenaar F, de Leede LG, Breimer DD. Rectal drug administration: clinical pharmacokinetic considerations. Clin Pharmacokinet. 1982 Jul-Aug;7(4):285-311. doi: 10.2165/00003088-198207040-00002. PMID: 6126289.
Fuchs, U., Leipnitz, Ch., Lippert, T.H.. The action of oxytocin on sperm motility in vitro experiments with bull spermatozoa. Clin. Exp. Obstet. Gynecol. 1989, 16(4), 95–102.
Gallup GG Jr, Burch RL, Platek SM. Does semen have antidepressant properties? Arch Sex Behav. 2002 Jun;31(3):289-93. doi: 10.1023/a:1015257004839. PMID: 12049024.
Gallup, G. G., & Burch, R. L. (2004). Semen Displacement as a Sperm Competition Strategy in Humans. Evolutionary Psychology, 2(1). https://doi.org/10.1177/147470490400200105
Hoffman BU, Baba Y, Griffith TN, Mosharov EV, Woo SH, Roybal DD, Karsenty G, Patapoutian A, Sulzer D, Lumpkin EA. Merkel Cells Activate Sensory Neural Pathways through Adrenergic Synapses. Neuron. 2018 Dec 19;100(6):1401-1413.e6. doi: 10.1016/j.neuron.2018.10.034. Epub 2018 Nov 8. PMID: 30415995; PMCID: PMC6347413.
Kanno H, Kurata S, Hiradate Y, Hara K, Yoshida H, Tanemura K. High concentration of dopamine treatment may induce acceleration of human sperm motility. Reprod Med Biol. 2022 Oct 1;21(1):e12482. doi: 10.1002/rmb2.12482. PMID: 36310655; PMCID: PMC9601866.
Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, O'Connor G, Grati M, Mittal J, Yan D, Eshraghi AA, Deo SK, Daunert S, Liu XZ. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J Cell Physiol. 2017 Sep;232(9):2359-2372. doi: 10.1002/jcp.25518. Epub 2017 Apr 10. PMID: 27512962; PMCID: PMC5772764.
Moore HD, Martin M, Birkhead TR. No evidence for killer sperm or other selective interactions between human spermatozoa in ejaculates of different males in vitro. Proc Biol Sci. 1999 Dec 7;266(1436):2343-50. doi: 10.1098/rspb.1999.0929. PMID: 10643078; PMCID: PMC1690463.
Nicole W. A question for women's health: chemicals in feminine hygiene products and personal lubricants. Environ Health Perspect. 2014 Mar;122(3):A70-5. doi: 10.1289/ehp.122-A70. PMID: 24583634; PMCID: PMC3948026.
Novella-Maestre E, Carda C, Ruiz-Sauri A, Garcia-Velasco JA, Simon C, Pellicer A. Identification and quantification of dopamine receptor 2 in human eutopic and ectopic endometrium: a novel molecular target for endometriosis therapy. Biol Reprod. 2010 Nov;83(5):866-73. doi: 10.1095/biolreprod.110.084392. Epub 2010 Jun 23. PMID: 20574053.
PARKER, G.A. (1970), SPERM COMPETITION AND ITS EVOLUTIONARY CONSEQUENCES IN THE INSECTS. Biological Reviews, 45: 525-567. https://doi.org/10.1111/j.1469-185X.1970.tb01176.x
Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res. 2019 Oct;97(10):1223-1241. doi: 10.1002/jnr.24476. Epub 2019 May 29. PMID: 31144383.
Pham, M.N., Jeffery, A.J., Sela, Y. et al. Duration of Cunnilingus Predicts Estimated Ejaculate Volume in Humans: a Content Analysis of Pornography. Evolutionary Psychological Science 2, 220–227 (2016). https://doi.org/10.1007/s40806-016-0057-5
Press MF, Nousek-Goebl NA, Bur M, Greene GL. Estrogen receptor localization in the female genital tract. Am J Pathol. 1986 May;123(2):280-92. PMID: 2939725; PMCID: PMC1888320.
Polakovičová S, Csöbönyeiová M, Filova B, Borovský M, Maršík L, Kvasilová A, Polák Š. Merkel-like cell distribution in the epithelium of the human vagina. An immunohistochemical and TEM study. Eur J Histochem. 2018 Feb 16;62(1):2836. doi: 10.4081/ejh.2018.2836. PMID: 29569875; PMCID: PMC5827109.
Ramírez AR, Castro MA, Angulo C, Ramió L, Rivera MM, Torres M, Rigau T, Rodríguez-Gil JE, Concha II. The presence and function of dopamine type 2 receptors in boar sperm: a possible role for dopamine in viability, capacitation, and modulation of sperm motility. Biol Reprod. 2009 Apr;80(4):753-61. doi: 10.1095/biolreprod.108.070961. Epub 2008 Dec 10. PMID: 19074002.
Ramírez-Reveco A, Villarroel-Espíndola F, Rodríguez-Gil JE, Concha II. Neuronal signaling repertoire in the mammalian sperm functionality. Biol Reprod. 2017 Mar 1;96(3):505-524. doi: 10.1095/biolreprod.116.144154. PMID: 28339693.
Schjenken JE, Robertson SA. The Female Response to Seminal Fluid. Physiol Rev. 2020 Jul 1;100(3):1077-1117. doi: 10.1152/physrev.00013.2018. Epub 2020 Jan 30. PMID: 31999507.
Suarez SS, Pacey AA. Sperm transport in the female reproductive tract. Hum Reprod Update. 2006 Jan-Feb;12(1):23-37. doi: 10.1093/humupd/dmi047. Epub 2005 Nov 4. PMID: 16272225.
Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018 Mar;14(3):133-150. doi: 10.1038/nrneurol.2017.188. Epub 2018 Jan 29. PMID: 29377008; PMCID: PMC5829048.
Taniya MA, Chung HJ, Al Mamun A, Alam S, Aziz MA, Emon NU, Islam MM, Hong SS, Podder BR, Ara Mimi A, Aktar Suchi S, Xiao J. Role of Gut Microbiome in Autism Spectrum Disorder and Its Therapeutic Regulation. Front Cell Infect Microbiol. 2022 Jul 22;12:915701. doi: 10.3389/fcimb.2022.915701. PMID: 35937689; PMCID: PMC9355470.
Wang H, Eriksson H, Sahlin L. Estrogen receptors alpha and beta in the female reproductive tract of the rat during the estrous cycle. Biol Reprod. 2000 Nov;63(5):1331-40. doi: 10.1095/biolreprod63.5.1331. PMID: 11058536.
Wang Y, Xie Z. Exploring the role of gut microbiome in male reproduction. Andrology. 2022 Mar;10(3):441-450. doi: 10.1111/andr.13143. Epub 2022 Jan 5. PMID: 34918486.
Watson CS, Alyea RA, Cunningham KA, Jeng YJ. Estrogens of multiple classes and their role in mental health disease mechanisms. Int J Womens Health. 2010 Aug 9;2:153-66. doi: 10.2147/ijwh.s6907. PMID: 21072308; PMCID: PMC2971739.
